Thousands of drugs are marketed throughout the world. The origins of these drugs fall into three
categories:
1. natural products—compounds isolated from natural sources,
such as plants or bacteria,
2. natural products that have been chemically modified in the
laboratory, or
3. synthetic compounds (made entirely in the laboratory).
Most drugs obtained from natural sources consist of a single enantiomer. It is important to realize that a pair of enantiomers will rarely exhibit the same potency. We have seen in previous
chapters that drug action is usually the result of drug-receptor binding. If the drug binds to the receptor in at least three places (called three-point binding), then one enantiomer of the drug may be more capable of binding with the receptor:
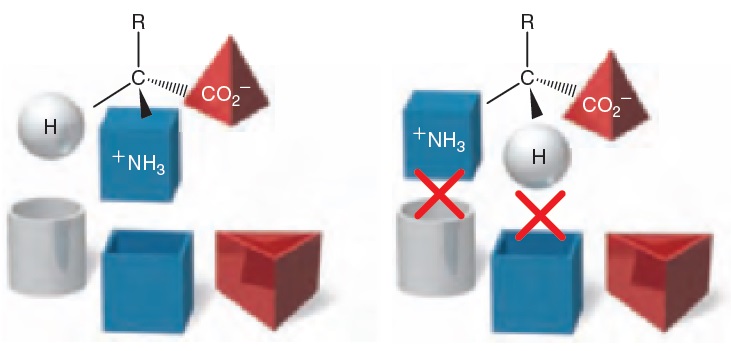
The first compound (left) can bind with the receptor, while its enantiomer (right) cannot bind with the receptor. For this reason, enantiomers will rarely produce the same biological response.
As an example, consider the enantiomers of ibuprofen:
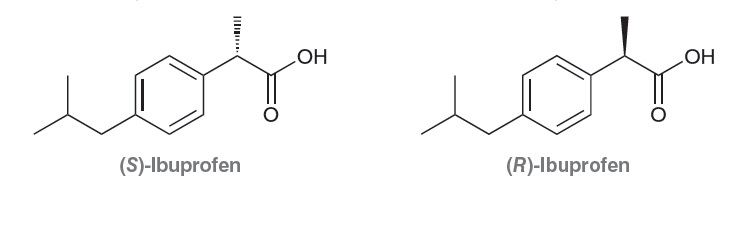
Ibuprofen is an analgesic (painkiller) with anti-inflammatory properties. The S enantiomer is the active agent, while the R enantiomer is inactive. Nevertheless, ibuprofen is sold as a
mixture of both enantiomers (under the trade names Advil and Motrin), because the benefit of separating the enantiomers is not clear. In fact, there is evidence that the human body is
capable of slowly converting the R enantiomer into the desired S enantiomer. Many synthetic drugs are sold as a mixture of enantiomers, because of the high cost and difficulty associated
with separating enantiomers. In many cases, enantiomers can trigger entirely different
physiological responses. For example, consider the enantiomers of Timolol:
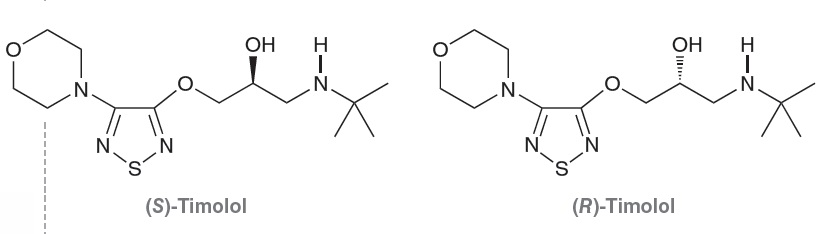
The S enantiomer treats angina and high blood pressure,while the R enantiomer is useful in treating glaucoma. In this example, both enantiomers produce desirable, albeit different,
results. In other cases, one enantiomer can produce an undesirable response. For example, consider the enantiomers of penicillamine:
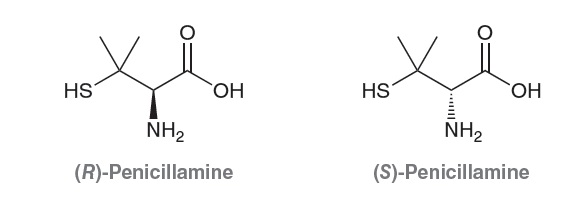
The S enantiomer was used to treat chronic arthritis (until other, more effective drugs were developed), while the R enantiomer is highly toxic. In such a case, the drug cannot be sold as a mixture of enantiomers.
Another example is naproxen:
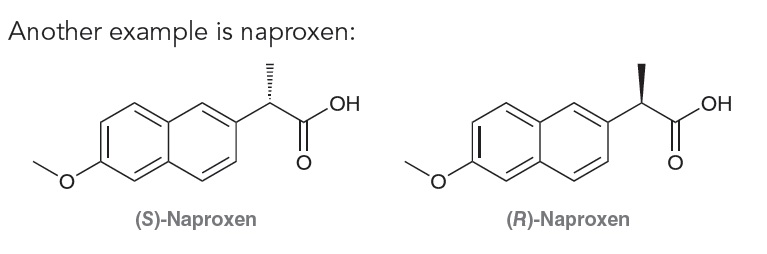
The S enantiomer is an anti-inflammatory agent, while the R enantiomer is a liver toxin. In these cases where one enantiomer of the drug is known to be a toxin, the drug is sold as a single enantiomer. However, the vast majority of drugs on the market are sold as mixtures of
enantiomers. The FDA has recently encouraged development of single enantiomers by
allowing drug companies to apply for new patents that cover single enantiomers of drugs previously sold as mixtures (provided that they demonstrate the advantages
of the single enantiomer over the mixture of enantiomers). Recent advances in enantioselective
synthesis (discussed in Section 8.8) have opened new doorways to the production of
single-enantiomer drugs. This is reflected by the fact that most new drugs entering the market
are sold as a single enantiomer. For more examples of enantiomers that produce
different biological responses.










Comments
Loading…