Ethanol is a primary alcohol. Therefore, ethanol will react with potassium dichromate in acidic conditions to produce acetic acid.
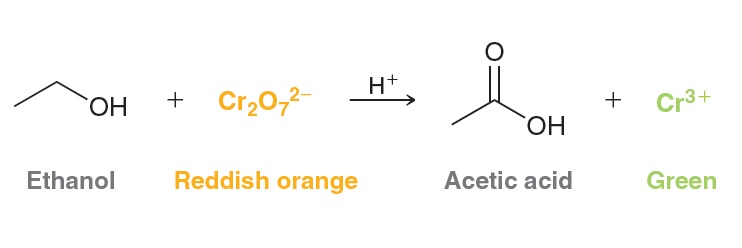
In the process, ethanol is oxidized, and the chromium reagent is reduced. The new chromium compound is a different color, and therefore, the progress of the reaction can be monitored by the color change from reddish-orange to green. This reaction formed the basis for many of the early breath tests that assessed the level of blood alcohol. Breath tests that utilize this reaction are still commercially available. The test consists of a tube, where one end is fitted with a mouthpiece and the other end has a bag. The inside of the tube contains sodium dichromate that is adsorbed onto the surface of an inert solid, such as silica gel. As the user blows air through the tube and fills up the bag, the alcohol in the user’s breath reacts with the oxidizing agent in the tube, and a color change occurs. The extent of the color change gives an indication of the blood alcohol content.
The Breathalyzer is based on the same idea, but it is more accurate. A measured volume of breath is bubbled through an aqueous acidic solution of potassium dichromate, and the change in color is measured with an ultraviolet-visible (UV-VIS) spectrophotometer (Section 17.11). Contrary to popular belief, it is not possible to beat the test by sucking a breath mint or rinsing with a breath freshener. These techniques might fool a person, but they won’t fool the potassium dichromate.









Comments
Loading…